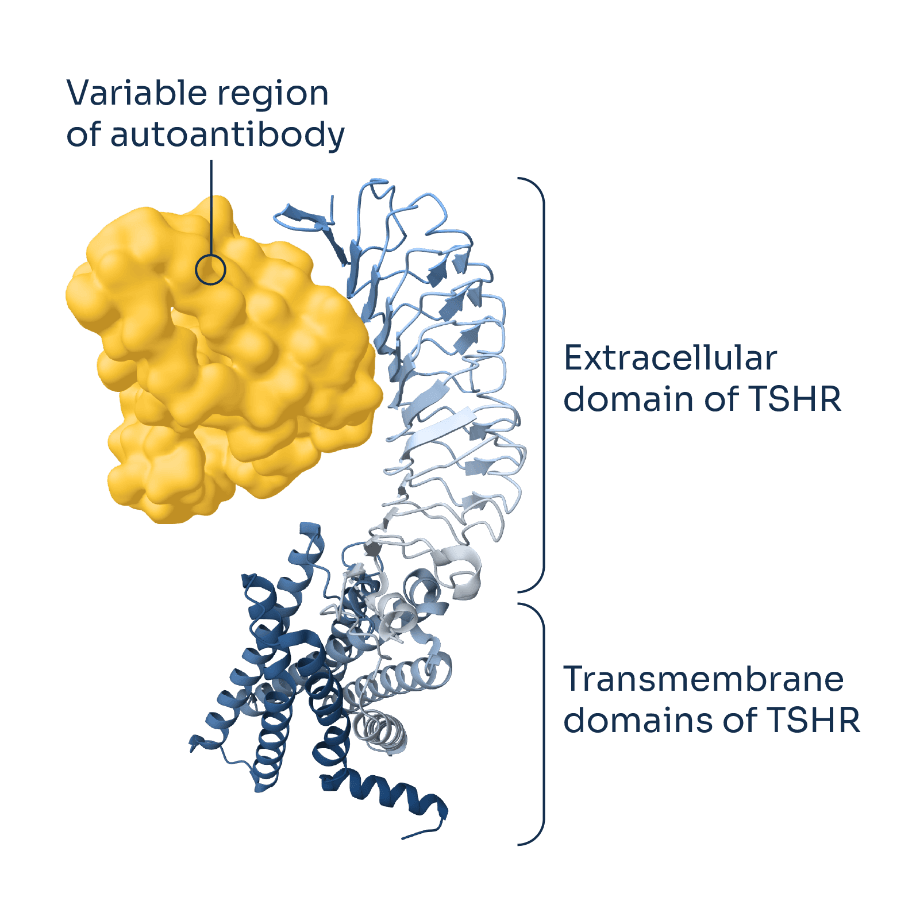
Graves’ disease is the most common cause of hyperthyroidism. It is driven by pathogenic autoantibodies that bind thyroid-stimulating hormone receptors (TSHR) in the thyroid gland, triggering excess thyroid hormone production and accelerating metabolic activity. These same autoantibodies also lead to Graves’ orbitopathy, or thyroid eye disease (TED), which affects approximately half of patients with Graves’ disease, and can cause ocular inflammation, pain, bulging of the eyes, and double vision.
Standard therapies for Graves’ disease have remained largely unchanged for decades and can be burdensome. Many patients ultimately require radioactive iodine treatment or thyroidectomy to shrink or remove the thyroid, resulting in loss of thyroid function and lifelong hormone replacement.
Merida is developing MER511, a precision biologic designed to selectively eliminate pathogenic autoantibodies and their B-cell sources while preserving normal immune function. By directly targeting the underlying drivers of Graves’ diseases, MER511 aims to directly target the autoantibodies that cause this difficult-to-manage disease, while sparing healthy thyroid signaling.
MER511 is being evaluated in the NEXUS Phase 1 Study for the treatment of Graves’ disease. Learn more about the NEXUS Study at ClinicalTrials.gov (NCT07305818).
For any inquiries on clinical trials at Merida, please contact clinicaltrials@meridabio.com.